NCERT Solutions for Class 9 Science Chapter 4 Structure of Atom
Looking for easy and reliable NCERT Solutions for Class 9 Science Chapter 4 Structure of Atom? Here you will find clear, step-by-step answers of all the NCERT solutions for Class 9 Science Chapter 4. These questions are from the latest NCERT books. In this article, you can get complete NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom in one place.
These solutions are part of NCERT Solutions for Class 9 Science.
For a better understanding of this chapter, you should also see Notes on Class 9 Science Chapter 4 Structure of the Atom.
NCERT Textbook Solution for Class 9 Science – Page 47
1. What are the canal rays?
Solution:
Canal rays are positively charged radiations produced in a gas discharge tube. These rays were discovered by E. Goldstein and are also known as anode rays. The study of canal rays helped in the identification of the positively charged subatomic particle, the proton.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Solution:
An atom with one electron and one proton will not carry any charge. The proton has a positive charge and the electron has an equal but opposite negative charge. These charges cancel each other, making the atom electrically neutral.
Class 9 Science NCERT Textbook – Page 49
1. On the basis of Thompson’s model of an atom, explain how the atom is neutral as a whole.
Solution:
According to Thomson’s model, an atom is a uniform sphere of positive charge with negatively charged electrons embedded in it. The total positive charge of the sphere is equal to the total negative charge of the electrons present. Since both charges are equal in magnitude and opposite in nature, they balance each other. As a result, the atom as a whole is electrically neutral.
2. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Solution:
According to Rutherford’s model, the nucleus of an atom contains protons, which are positively charged subatomic particles.
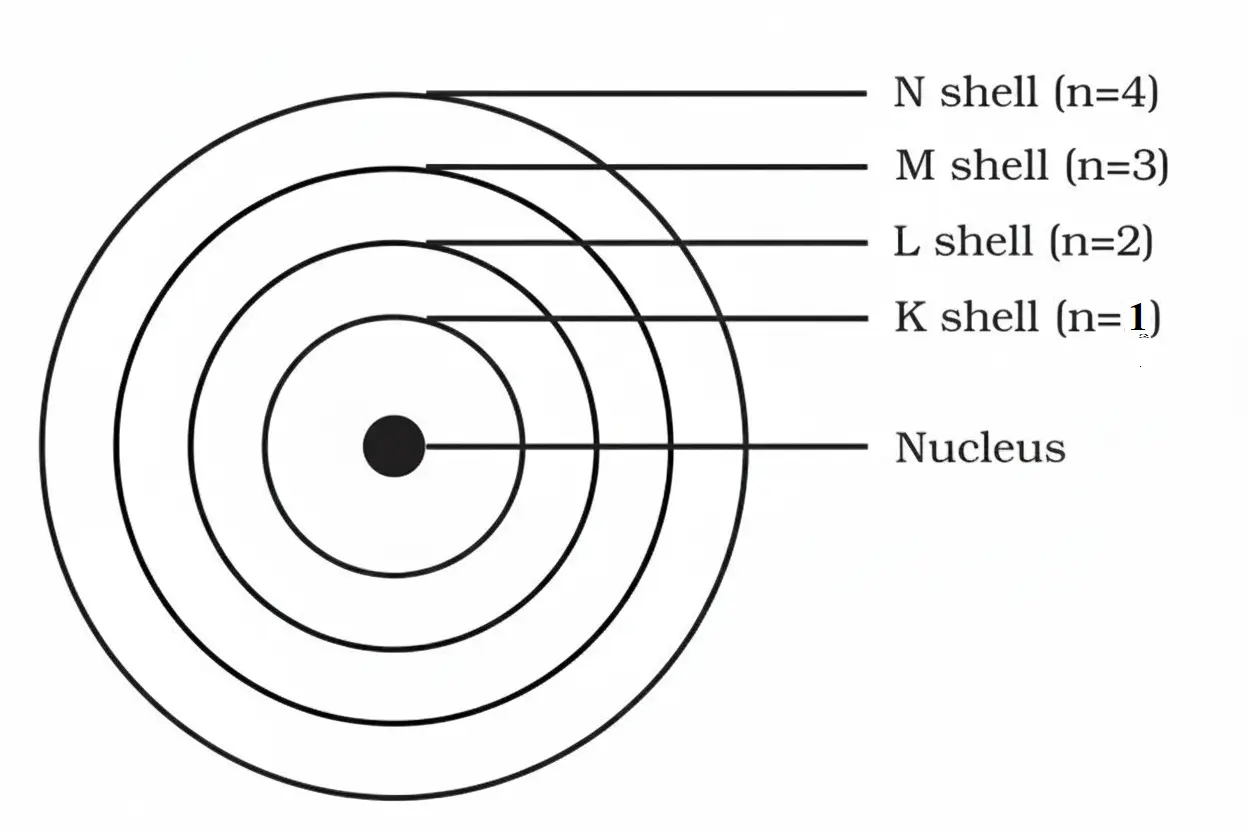
3. Draw a sketch of Bohr’s model of an atom with three shells.
Solution:
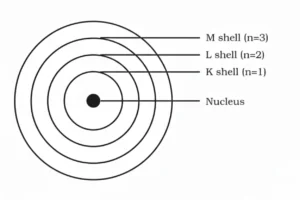
4. What do you think would be the observation if the α–particle scattering experiment is carried out using a foil of a metal other than gold?
Solution:
If the α-particle scattering experiment is performed using a metal foil other than gold, the basic observations would remain the same. This is because the internal structure of atoms is similar across different elements.
However, obtaining a foil as thin as gold is difficult for most metals. Using a thicker foil may cause more α-particles to bounce back, making it harder to accurately determine the distribution of positive charge in the atom.
Class 9 Science NCERT Textbook – Page 49
1. Name the three subatomic particles of an atom.
Solution:
An atom consists of three subatomic particles:
- Protons – Positively charged
- Electrons – Negatively charged
- Neutrons – Neutral in nature (no charge)
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Solution:
The atomic mass of an atom is the sum of the number of protons and neutrons in its nucleus. A helium atom has an atomic mass of 4 u and contains 2 protons. Therefore, the number of neutrons = 4 – 2 = 2. Hence, a helium atom has 2 neutrons.
NCERT Textbook for Class 9 Science – Page 50
1. Write the distribution of electrons in Carbon and Sodium atoms.
Solution:
The electron distribution in a carbon atom, which has 6 electrons, is as follows:
- K-shell (first orbit): 2 electrons
- L-shell (second orbit): 4 electrons
This can also be written as 2, 4.
The electron distribution in a sodium atom, which has 11 electrons, is:
- K-shell (first orbit): 2 electrons
- L-shell (second orbit): 8 electrons
- M-shell (third orbit): 1 electron
This can also be written as 2, 8, 1.
2. If the K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Solution:
The K shell can accommodate a maximum of 2 electrons, while the L shell can hold up to 8 electrons. If both shells are completely filled, then the total number of electrons in the atom would be (8 + 2)= 10 electrons.
Class 9 Science NCERT Textbook – Page 52
1. How will you find the valency of chlorine, sulphur and magnesium?
Solution:
The valency of an element is determined by the number of electrons in its outermost shell and its tendency to complete an octet.
- Chlorine has the electronic configuration 2, 8, 7. Since it has more than 4 electrons in its outermost shell, its valency is calculated as 8 − 7 = 1.
- Sulphur has the electronic configuration 2, 8, 6. As it also has more than 4 electrons in the outermost shell, its valency is 8 − 6 = 2.
- Magnesium has the electronic configuration 2, 8, 2. Because it has fewer than 4 electrons in its outermost shell, its valency is equal to the number of outermost electrons, which is 2.
NCERT Textbook for Class 9 Science – Page 52
1. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) What is the atomic number of the atom? and
(ii) What is the charge on the atom?
Solution:
(i) The atomic number of an atom is equal to the number of protons present in its nucleus. Since the atom has 8 protons, its atomic number is 8.
(ii) Since the number of both electrons (-ve) and protons ( +ve ) is equal, therefore, the charge on the atom is 0 and it is a neutral atom
2. With the help of given Table 4.1, find out the mass number of oxygen and sulphur atom.
Table: Composition of Atoms of the First Eighteen Elements with Electron Distribution in Various Shells.
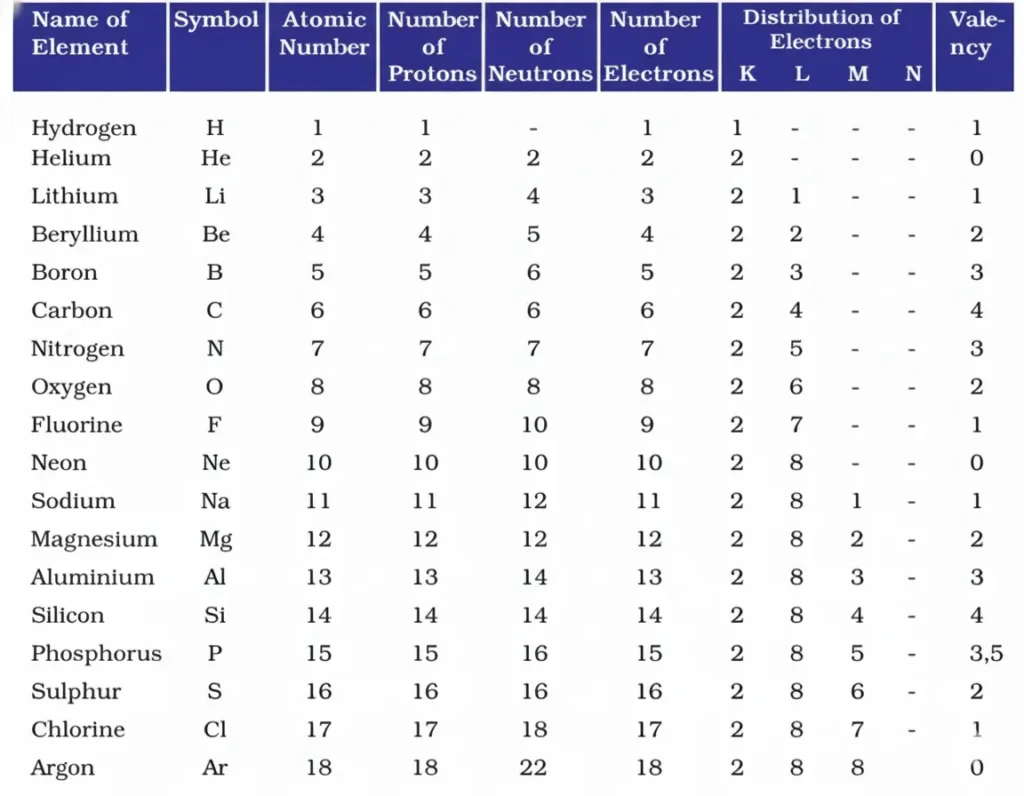
Solution:
(a) To find the mass number of Oxygen,
Number of protons = 8
Number of neutrons = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, the mass number of oxygen = 16
(b) To find the mass number of Sulphur,
Number of protons = 16
Number of neutrons = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
NCERT Solutions for Class 9 Science Chapter 4 Textbook – Page 53
1. For the symbols H, D and T, tabulate three subatomic particles found in each of them.
Solution:
The symbols H, D, and T represent three isotopes of hydrogen. The number of subatomic particles present in each is given below:
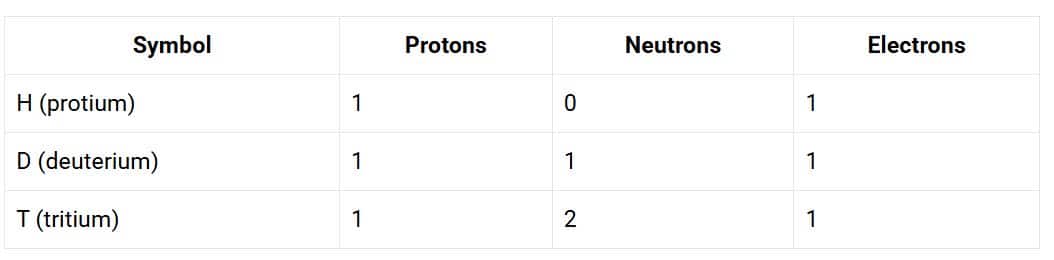
2. Write the electronic configuration of any one pair of isotopes and isobar.
1. Solution:
Two isotopes of carbon are:
\( \text{Carbon-12: } ({}^{12}_{6}\text{C})
\qquad
\text{Carbon-14: } ({}^{14}_{6}\text{C}) \)
Both have the same atomic number, so their electronic configuration is: 2, 4
Two isobars of carbon are:
\( \text{Calcium-40: } ({}^{40}_{20}\text{Ca})
\qquad
\text{Argon-40: } ({}^{40}_{18}\text{Ar}) \)
- Both have the same mass number but different atomic numbers.
- Calcium-40 electronic configuration: 2, 8, 8, 2
- Argon-40 electronic configuration: 2, 8, 8
Questions from NCERT Text Book Page: 54
1. Compare the properties of electrons, protons and neutrons.
Solution:
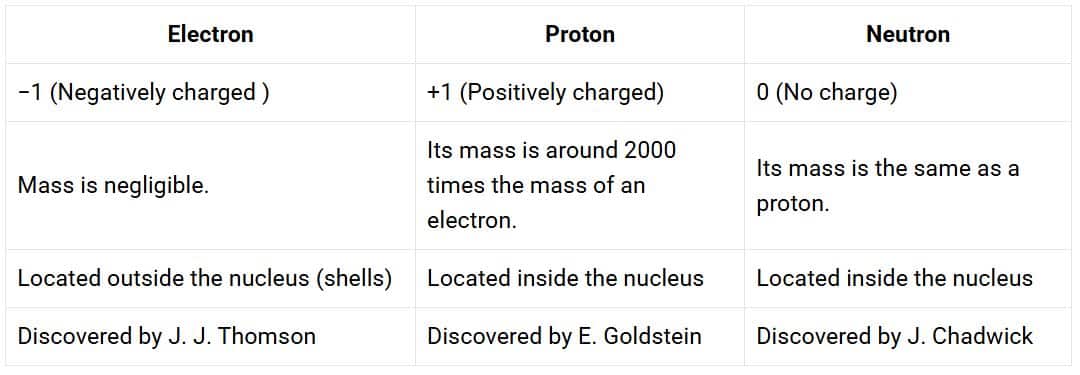
2. What are the limitations of J.J. Thomson’s model of the atom?
Solution:
The limitations of J. J. Thomson’s model of the atom are:
- It failed to explain the results of Rutherford’s alpha-particle scattering experiment.
- It could not explain why most alpha particles passed straight through the foil while some were deflected or rebounded.
- It did not describe the presence of a nucleus of an atom.
- It failed to explain the stability of the atom and the proper arrangement of electrons within it.
3. What are the limitations of Rutherford’s model of the atom?
Solution:
Rutherford’s model of the atom could not explain the stability of atoms. According to this model, electrons revolve around the nucleus in circular orbits. Any particle moving in a circular path experiences acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were true, atoms would be highly unstable and matter would not exist in the stable form observed in nature. However, since we know that atoms are quite stable, Rutherford’s model fails to account for this stability.
4. Describe Bohr’s model of the atom.
Solution:
Bohr’s model of the atom states that–
- An atom has a nucleus at its centre.
- Negatively charged electrons revolve around the positively charged nucleus.
- Electrons move in certain fixed orbits called discrete orbits or energy levels.
- While revolving in these orbits, electrons do not radiate energy.
- These orbits or shells are represented by the letters K, L, M, N or the numbers n = 1, 2, 3, 4
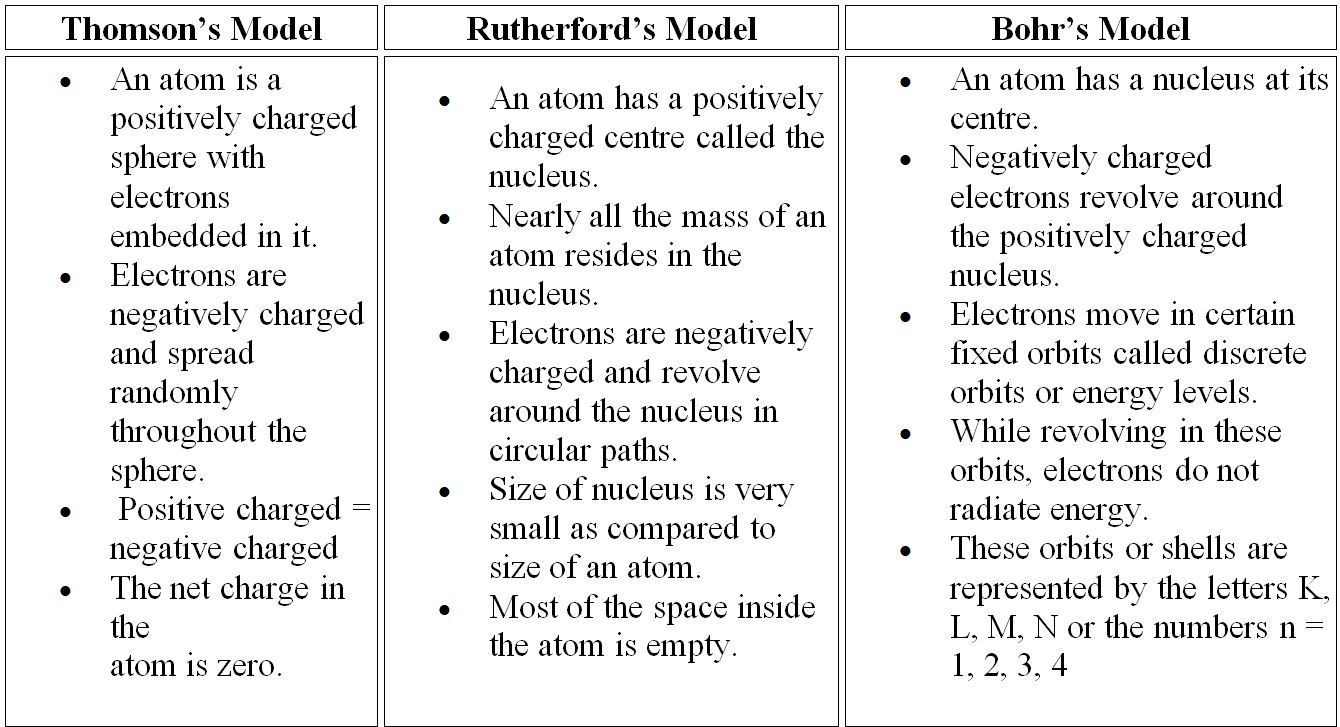
5. Compare all the proposed models of an atom given in this chapter.
Solution:
6. Summarise the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.
Solution:
The rules for writing the distribution of electrons in various shells for the first eighteen elements are:
The maximum number of electrons a shell can hold is given by the formula 2n², where n is the shell number (1, 2, 3…).
K shell (n = 1): 2 × (1)² = 2 electrons
L shell (n = 2): 2 × (2)² = 8 electrons
M shell (n = 3): 2 × (3)² = 18 electrons
N shell (n = 4): 2 × (4)² = 32 electrons
The outermost shell can hold a maximum of 8 electrons.
Electrons are filled into shells in a stepwise manner, with the inner shells being
filled first, followed by the outer shells.
9. Define valency by taking examples of silicon and oxygen.
Solution:
Valency is the combining capacity of an atom. The valency of an element is determined by the number of electrons in its outermost shell.
If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons.
For silicon (atomic number 14), the electronic configuration is K – 2, L – 8, M – 4. The outermost shell has 4 electrons. Thus, the valency of silicon is 4.
On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight.
For oxygen (atomic number 8), the electronic configuration is K – 2, L – 6. The outermost shell has 6 electrons. Thus, the valency of oxygen is: 8 – 6 = 2.
Thus, the valency of silicon is 4, and the valency of oxygen is 2.
10. Explain with examples
(i) Atomic number,
(ii) Mass number,
(iii) Isotopes and
(iv) Isobars.
Give any two uses of isotopes.
Solution:
(i) Atomic number: The atomic number of an element is the total number of protons present in the nucleus of its atom. For example, oxygen has 8 protons, so its atomic number is 8.
(ii) Mass number: The mass number of an atom is the sum of the total number of protons and neutrons in its nucleus. For example, oxygen has 8 protons and 8 neutrons, so its mass number is 16.
(iii) Isotopes: Isotopes are atoms of the same element that have the same atomic number but different mass numbers. Example: Hydrogen has three isotopes—protium (¹H), deuterium (²H), and tritium (³H).
(iv) Isobars: Isobars are atoms of different elements that have the same mass number but different atomic numbers. Example: Calcium (₂₀⁴⁰Ca) and Argon (₁₈⁴⁰Ar) are isobars.
Uses of isotopes:
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goitre.
9. Na+ has completely filled K and L shells. Explain.
Ans:
The atomic number of sodium is 11, so a neutral sodium atom has 11 electrons. So, its electronic configuration is 2, 8, 1. When a sodium atom (Na) loses 1 electron and forms a Na⁺ ion, the number of electrons becomes 10 and the new electronic configuration is 2, 8. Thus, in the Na⁺ ion, both the K shell (2 electrons) and L shell (8 electrons) are completely filled, which makes the ion stable.
10. If bromine atom is available in the form of say, two isotopes 7935Br (49.7%) and 8135Br (50.3%), calculate the average atomic mass of bromine atom.
Answer:
We know that, \( \text{Average atomic mass} = \frac{(m_1 \times p_1) + (m_2 \times p_2)}{100} \)
So, Average atomic mass of bromine
\( = \frac{(79 \times 49.7) + (81 \times 50.3)}{100} \)
\( = \frac{3926.3 + 4074.3}{100} \)
\( = \frac{8000.6}{100} \)
= 80.0 u (approximately)
11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 188X in the sample?
Answer:
Let the percentage of isotope \( ^{16}_{8}X \) be \( x\% \)
Then the percentage of isotope \( ^{18}_{8}X \) will be \( (100 – x)\% \)
The average atomic mass is given as 16.2 u.
\(∴ \frac{(16 \times x) + [18 \times (100 – x)]}{100} = 16.2 \)
\(⇒ 16x+1800−18x=1620 \)
\(⇒ −2x=−180 \)
\(∴ x=90 \)
So, the percentage of \( ^{16}_{8}X \) is 90%, and the percentage of \( ^{18}_{8}X \) is 10%.
12. If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
If the atomic number Z=3, the element has three electrons.
Its electronic configuration is 2, 1.
Since the outermost shell has only one electron.
Therefore, the valency of the element is 1.
The element with atomic number 3 is lithium (Li).
13. Composition of the nuclei of two atomic species X and Y are given as under
| X | Y | |
| Protons = | 6 | 6 |
| Neutrons = | 6 | 8 |
Give the mass number of X and Y. What is the relation between the two species?
Ans: Mass number = Number of protons + Number of neutrons
Mass number of X = 6 + 6 = 12.
Mass number of Y = 6 + 8 = 14.
Both X and Y have the same number of protons, which means they have the same atomic number. However, their mass numbers are different due to a different number of neutrons. Therefore, X and Y are isotopes of the same element.
16. For the following statements, write T for true and F for false.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of a proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Solution:
(a) F – J.J. Thomson proposed that the atom is a positively charged sphere with electrons embedded in it; he did not propose the concept of a nucleus.
(b) F – A neutron is not formed by combining a proton and an electron; it is a fundamental subatomic particle with no net charge.
(c) T – The mass of an electron is approximately 1/20001/20001/2000 the mass of a proton.
(d) F – An isotope of iodine is used in medical treatments, such as for thyroid disorders, but tincture of iodine is made from elemental iodine, not its isotopes.
Put tick against correct choice and cross (x) against wrong choice in questions 15, 16 and 17.
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(c) Proton
(b)Electron
(d)neutron
Answer: (a) Atomic nucleus
16. Isotopes of an element have
(a) the same physical properties
(c) different number of neutrons
(b)different number of neutrons
(d) different atomic numbers.
Answer: (c) different number of neutrons
17. Number of valence electrons in Cl⁻ ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer: (b) 8
18. Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Answer: (d) 2, 8, 1
19. Complete the following table.
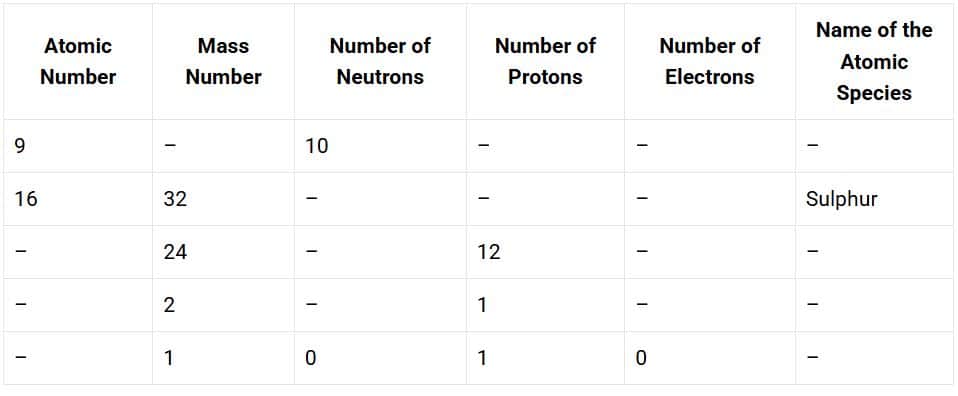
Solution:
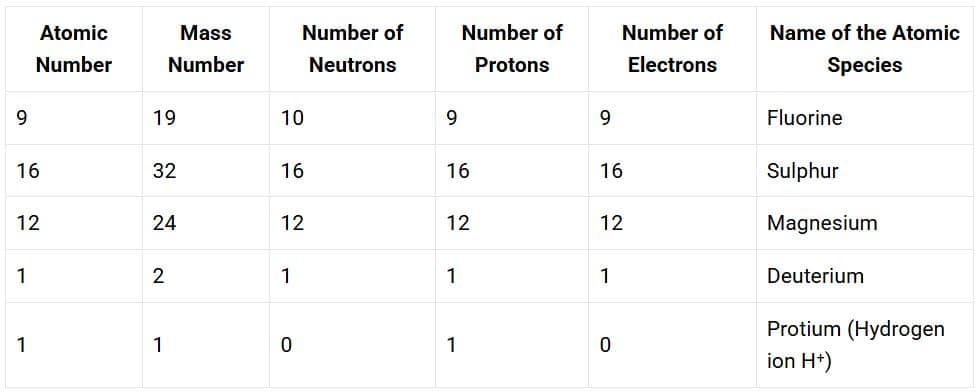
NCERT Solutions for Class 9 Science Chapter 1 Matter In Our Surroundings
NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
NCERT Solutions for Class 9 Science Chapter 3 Atoms And Molecules
NCERT Solutions for Class 9 Science Chapter 4 Structure Of The Atom
NCERT Solutions for Class 9 Science Chapter 5 The Fundamental Unit Of Life
NCERT Solutions for Class 9 Science Chapter 6 Tissues
NCERT Solutions for Class 9 Science Diversity In Living Organism
NCERT Solutions for Class 9 Science Chapter 8 Motion
NCERT Solutions for Class 9 Science Chapter 9 Force And Laws Of Motion
NCERT Solutions for Class 9 Science Chapter 10 Gravitation
NCERT Solutions for Class 9 Science Chapter 11 Work And Energy
NCERT Solutions for Class 9 Science Chapter 12 Sound
NCERT Solutions for Class 9 Science Chapter 13 Why Do We Fall ill
NCERT Solutions for Class 9 Science Chapter 14 Natural Resources
NCERT Solutions for Class 9 Science Chapter 15 Improvement In Food Resources
