NCERT Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure
| Welcome to the complete NCERT Solutions for Class 9 Science Chapter 2 – Is Matter Around Us Pure?This page provides accurate answers, step-by-step explanations, images, and easy-to-understand solutions to all the NCERT textbook questions from this chapter. If you’re a Class 9 student studying with the NCERT Science textbook, you’ve definitely come across this important chapter. Here, you’ll find everything you need in one place. Perfect for CBSE and State Board exam preparation!For a better understanding of this chapter, you should also see the Summary, Notes & Key Concepts of this Chapter Class 9 Science Chapter 2: Is Matter Around Us Pure? – Summary, Notes & Key Concepts For MCQs from this chapter Click Below |
NCERT Textbook for Class 9 Science – Page 15
Question 1. What is meant by a substance?
Answer:-
A substance is a pure form of matter that has the same composition and properties throughout its mass. It can be a single element or a compound.
There are two types of substances:
Elements – It is made of only one type of atom. e.g., iron
Compounds – It is made of two or more elements chemically combined in a fixed ratio. e.g., carbon dioxide.
Question 2. List the points of differences between homogeneous and heterogeneous mixtures.
Answer:
Difference Between Homogeneous and Heterogeneous Mixtures
| Homogeneous Mixture | Heterogeneous Mixture |
|---|---|
| A homogeneous mixture has a uniform composition throughout its mass. | A heterogeneous mixture has a non-uniform composition throughout its mass. |
| The entire mixture looks the same in all parts. | The mixture looks different in different parts. |
| The particles are not visible to the naked eye. | The particles are often visible to the naked eye. |
| It consists of only one phase. | It consists of two or more phases. |
| Components cannot be separated easily by physical methods. | Components can be separated easily by physical methods. |
| Salt in water, sugar in water, air, and alloys are homogeneous mixtures. | Oil and water, sand and iron, salad, and soil are heterogeneous mixtures. |
Class 9 Science NCERT Textbook Page 18
Question 1. Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer: See above
Question 2. How are sol, solution and suspension different from each other?
Answer:
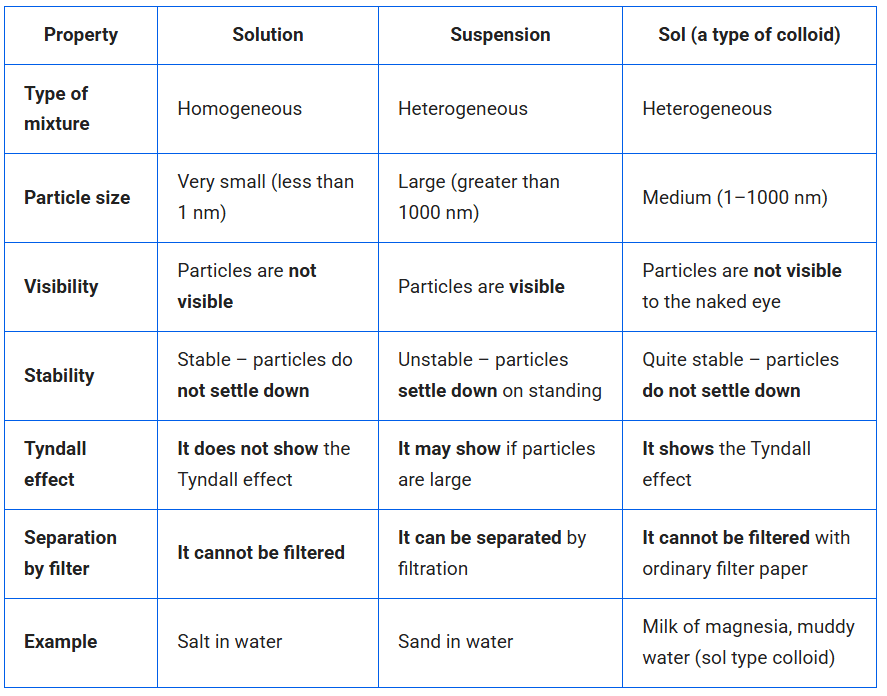
3. To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Given:
Mass of sodium chloride (solute) = 36 g
Mass of water (solvent) = 100 g
So, Mass of solution = 36 g + 100 g = 136 g
We know, Concentration = (Mass of solute / Mass of solution) × 100
Concentration = (36 g / 136 g) × 100
Concentration = 26.47%
Class 9 Science NCERT Textbook Page 24
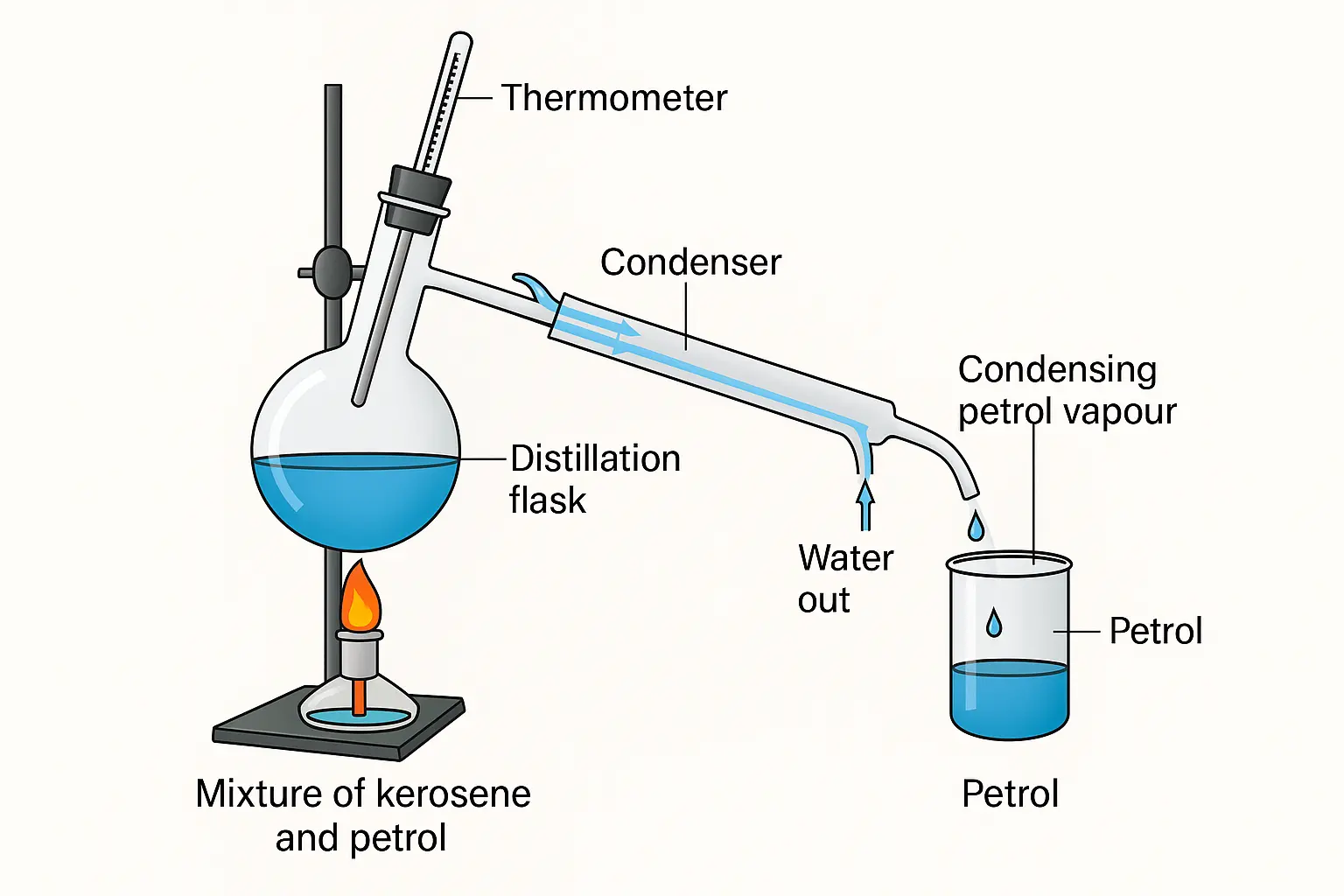
1. How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer:
To separate a mixture of kerosene and petrol, which are miscible liquids with a boiling point difference of more than 25°C, we use the method of simple distillation.
Steps for Separation by Simple Distillation:
Take the mixture in a distillation flask.
Heat the mixture slowly.
Petrol, having a lower boiling point, will vaporise first.
The vapour passes through the condenser, where it cools and condenses into liquid.
Collect petrol in a separate container.
Kerosene remains in the flask as it has a higher boiling point.
2. Name the technique to separate
(i) butter from curd,
(ii) salt from sea-water,
(iii) camphor from salt.
Answer:
Here are the correct techniques to separate each mixture:
Butter from curd – Centrifugation
Salt from sea-water – Evaporation
Camphor from salt – Sublimation
3. What type of mixtures are separated by the technique of crystallisation?
Answer:
The technique of crystallisation is used to separate solids that are dissolved in a liquid.
NCERT Textbook Questions Page 24
Question 2. Classify the following as chemical or physical changes:
- cutting of trees,
- melting of butter in a pan,
- rusting of almirah,
- boiling of water to form steam,
- passing of electric current, through water and the water breaking down into hydrogen and oxygen gas,
- dissolving common salt in water,
- making a fruit salad with raw fruits and
- burning of paper and wood.
Answer:-
| Change | Type of Change |
|---|---|
| 1. Cutting of trees | Physical change |
| 2. Melting of butter in a pan | Physical change |
| 3. Rusting of almirah | Chemical change |
| 4. Boiling of water to form steam | Physical change |
| 5. Passing of electric current through water, breaking it into hydrogen and oxygen | Chemical change |
| 6. Dissolving common salt in water | Physical change |
| 7. Making a fruit salad with raw fruits | Physical change |
| 8. Burning of paper and wood | Chemical change |
3. Try segregating the things around you as pure substances or mixtures
Answer:
Pure Substances: Water, Iron, Oxygen, Gold, Salt, Sugar, etc.
Mixtures: Air, Milk, Soft drink, Soil, Salt solution, Fruit salad
Questions From the NCERT Textbook for Class 9 Science: Exercises
Question 1. Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Answer:
| Mixture to be separated | Best separation technique |
|---|---|
| (a) Sodium chloride from its solution in water | Evaporation / Crystallisation |
| (b) Ammonium chloride + sodium chloride | Sublimation |
| (c) Small metal pieces in engine oil | Filtration |
| (d) Different pigments from flower-petal extract | Paper chromatography |
| (e) Butter from curd | Centrifugation |
| (f) Oil from water | Separating funnel |
| (g) Tea leaves from tea | Filtration (straining) |
| (h) Iron pins from sand | Magnetic separation |
| (I) Wheat grains from husk | Winnowing |
| (j) Fine mud particles suspended in water | Sedimentation and Filtration |
2. Write the steps you would use for making tea. Use the words, solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer: Steps to Make Tea Using Scientific Terms
Boil water in a pan. Here, water acts as the solvent.
Add tea leaves and sugar to the hot water. Both are solutes.
Sugar dissolves in water because it is soluble, forming a solution.
Tea leaves are insoluble, so they do not dissolve in water.
Boil for a few minutes to extract flavour and colour from the tea leaves.
Add milk (optional), which also forms a solution with the mixture.
Pour the tea through a strainer to separate the tea leaves.
The liquid that passes through is called the filtrate.
The tea leaves left on the strainer are called the residue.
3. Pragya tested the solubility of three different substances at different temperatures and collected, the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).

(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe us the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
(a)
Solubility of potassium nitrate at 313 K = 62 g per 100 g of water
Mass of potassium nitrate in 50 g of water at 313 K= (62 / 100) × 50=31 g
(b) As we know, when the temperature decreases, the solubility of potassium chloride also decreases. As the saturated solution of potassium chloride cools from 353 K to room temperature, Pragya will observe that some of the dissolved potassium chloride begins to crystallize and settle down at the bottom.
(c)
Solubility of each salt at 293 K:
Potassium nitrate = 32 g
Sodium chloride = 36 g
Potassium chloride = 35 g
Ammonium chloride = 37 g
At 293 K, ammonium chloride has the highest solubility (37 g/100 g water).
(d)
As temperature increases, the solubility of most salts increases.
For example, potassium nitrate’s solubility increases from 21 g at 283 K to 167 g at 353 K.
4. Explain the following giving examples:
(a) Saturated solution
(b) Pure substance
(c) Colloid
(d) Suspension
Answer:
(a) Saturated Solution:
A saturated solution is one in which no more solute can be dissolved at a given temperature.
Example: If 36 g of salt is dissolved in 100 g of water at 293 K and no more salt can dissolve, then the solution is saturated.
(b) Pure Substance:
A pure substance has only one kind of particle and the same composition throughout. It has fixed physical and chemical properties.
Example: gold, silver, oxygen, etc, are pure substances.
(c) Colloid:
A colloid is a heterogeneous mixture in which the particles are small and uniformly spread. They do not settle down and scatter light (Tyndall effect).
Example: Ink, blood, milk, fog, and jelly are colloids.
(d) Suspension:
A suspension is a heterogeneous mixture where solid particles are suspended in a liquid. The particles are visible and settle down on standing.
Example: Sand in water, chalk powder in water.
5. Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air, soil, vinegar, filtered tea.
Answer:
Homogeneous: Soda water, vinegar, filtered tea.
Heterogeneous: Wood, air, soil.
6. How would you confirm that a colourless liquid given to you is pure water?
Answer:
We can confirm that a colourless liquid is pure water by checking its boiling point. Pure water boils at a fixed temperature of 100°C at atmospheric pressure. If the given liquid boils exactly at 100°C, it is pure water. If it boils at a temperature higher or lower than 100°C, it is not pure water and contains impurities.
7. Which of the following materials fall in the category of a “pure substance”?
(a) Ice (b) Milk (c) Iron (d) Hydrochloric acid (e) Calcium oxide (f) Mercury (g) Back (h) Wood (i) Air.
Answer:
The following fall under the category of pure substances:
✅ Pure Substances:
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
❌ Not Pure Substances (Mixtures):
(b) Milk (colloid)
(g) Brick (mixture of materials)
(h) Wood (heterogeneous mixture)
(i) Air (homogeneous mixture of gases)
8. Identify the solutions among the following mixtures.
(a) Soil (b) Sea water (c) Air (d) Coal (e) Soda water.
Answer:
The following are solutions (homogeneous mixtures):
✅ Solutions:
(b) Sea water
(c) Air
(e) Soda water
❌ Not Solutions (Heterogeneous Mixtures):
(a) Soil
(d) Coal
9. Which of the following will show “Tyndall effect”?
(a) Salt solution (b) Milk (c) Copper sulphate solution (d) Starch solution.
Answer:
The following will show the Tyndall effect:
✅ Show Tyndall Effect:
(b) Milk (colloid)
(d) Starch solution (colloid)
❌ Do Not Show Tyndall Effect (True Solutions):
(a) Salt solution
(c) Copper sulphate solution
10. Classify the following into elements, compounds and mixtures.
(a) Sodium (b) Soil (c) Sugar solution (d) Silver (e) Calcium carbonate (f) Tin (g) Silicon (h) Coal (i) Air (j) Soap (k) Methane (l) Carbon dioxide (m) Blood
Answer:
Classification into Elements, Compounds, and Mixtures
| Substance | Type |
|---|---|
| (a)Sodium | Element |
| (b) Soil | Mixture |
| (c) Sugar solution | Mixture (solution) |
| (d) Silver | Element |
| (e) Calcium carbonate | Compound |
| (f) Tin | Element |
| (g) Silicon | Element |
| (h) Coal | Mixture |
| (i) Air | Mixture |
| (j) Soap | Mixture (if commercial) / Compound (if pure) |
| (k) Methane | Compound |
| (l) Carbon dioxide | Compound |
| (m) Blood | Mixture (colloid) |
11. Which of the following are chemical changes?
(a) Growth of a plant (b) Rusting of iron (c) Mixing of iron filings and sand (d) Cooking of food (e) Digestion of food (f) Freezing of water (g) Burning of a candle.
Answer:
The following are chemical changes:
✅ Chemical Changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion of food
(g) Burning of a candle
❌ Not Chemical Changes (Physical Changes):
(c) Mixing of iron filings and sand (no new substance formed)
(f) Freezing of water (only change of state)
NCERT Solutions for Class 9 Science Chapter 1 Matter In Our Surroundings
NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure?
NCERT Solutions for Class 9 Science Chapter 3 Atoms And Molecules
NCERT Solutions for Class 9 Science Chapter 4 Structure Of The Atom
NCERT Solutions for Class 9 Science Chapter 5 The Fundamental Unit Of Life
NCERT Solutions for Class 9 Science Chapter 6 Tissues
NCERT Solutions for Class 9 Science Diversity In Living Organism
NCERT Solutions for Class 9 Science Chapter 8 Motion
NCERT Solutions for Class 9 Science Chapter 9 Force And Laws Of Motion
NCERT Solutions for Class 9 Science Chapter 10 Gravitation
NCERT Solutions for Class 9 Science Chapter 11 Work And Energy
NCERT Solutions for Class 9 Science Chapter 12 Sound
NCERT Solutions for Class 9 Science Chapter 13 Why Do We Fall ill
NCERT Solutions for Class 9 Science Chapter 14 Natural Resources
NCERT Solutions for Class 9 Science Chapter 15 Improvement In Food Resources
