Matter in Our Surroundings–NCERT Class 9 Science Chapter 1–Summary & Revision Notes
This article provides a complete summary and revision notes on ” Matter in Our Surroundings–NCERT Class 9 Science Chapter 1″ for the latest NCERT books. This chapter introduces the basic concepts of matter—its physical nature, states, properties, and changes under different conditions. Whether you’re preparing for CBSE or State Board exams, this detailed summary and revision notes will help you grasp the core concepts easily and confidently. These notes are also helpful for solving theoretical questions and MCQs from the textbook and school tests. For Chapter Questions & Answers of this chapter, you should also see For MCQs from this chapter, you should also see. |
1. Introduction to Matter in Our Surroundings
- Everything in the universe is made of matter. It includes air, food, water, stones, plants, animals, and stars. etc.
- Matter is anything that occupies space and has mass.
- Ancient Indian philosophers classified matter into five elements — the “Panch Tatva” – Air, Earth, Fire, Sky, and Water.
- Modern scientists classify matter based on physical properties and chemical nature.
- This chapter focuses on the physical properties of matter.
1.1 Physical Nature of Matter
1.1.1 Matter is Made Up of Particles
Activity 1.1 – Dissolving salt or sugar in water:
- When sugar or salt is added to water, it disappears, but the water level does not rise.
- This shows that particles of one substance go into the spaces between particles of another.
- So we can conclude that Matter is made up of particles, and these particles have space between them.
1.1.2 How Small are Particles of Matter?
- Particles of matter are very small beyond human imagination.
Activity 1.2 – Potassium permanganate in water:
A few crystals of potassium permanganate are added to water.
The solution is diluted 5 to 8 times, and still shows colour.
This shows that each crystal contains millions of small particles that keep dividing into smaller particles.
Similar activity can be done using Dettol – even when diluted, the smell remains even on repeated dilution.
So we can conclude that Particles of matter are very small, beyond our imagination.
1.2 Characteristics of Particles of Matter
1.2.1 Particles of Matter Have Space Between Them
Activities 1.1 and 1.2 conclude that:
- When substances like salt, sugar, potassium permanganate, or Dettol are dissolved in water, they mix completely without increasing the water level.
- This shows that the particles of matter have enough space between them, and one type of matter get into the spaces between particles of the other.
1.2.2 Particles of Matter are Continuously Moving
Activity 1.3 – Incense stick (agarbatti):
When an unlit stick is placed, we do not smell it from far.
When lit, we can smell it even from a distance.
This shows that particles are continuously moving.
Activity 1.4 – Ink and honey in water:
Ink spreads faster in water, while honey spreads slowly.
This shows that particles move and intermix on their own.
Activity 1.5 – Copper sulphate in hot and cold water:
Copper sulphate dissolves faster in hot water than in cold water because heat increases particle movement.
From the above three activities (1.3, 1.4 and 1.5), we can conclude the following:
Particles of matter are continuously moving, which means they have kinetic energy.
- As the temperature increases, the kinetic energy of the particles also increases.
- The intermixing of particles of two different substances is called diffusion.
- Diffusion happens faster at higher temperatures.
1.2.3 Particles of Matter Attract Each Other
Activity 1.6, 1.7, 1.8
Stronger human chains are harder to break.
- Iron nails are hard to break (strong attraction), chalk breaks easily (weaker attraction), and rubber stretches (flexible attraction).
We can’t cut water easily because a strong force of attraction holds its particles together.
From the above three activities (1.6, 1.7, 1.8), we can conclude the following:
Particles of matter have a force of attraction between them.
This force keeps particles together.
The strength of this force is different in different types of matter:
Strongest in solids
Less in liquids
Weak in gases
1.3 States of Matter – Summary
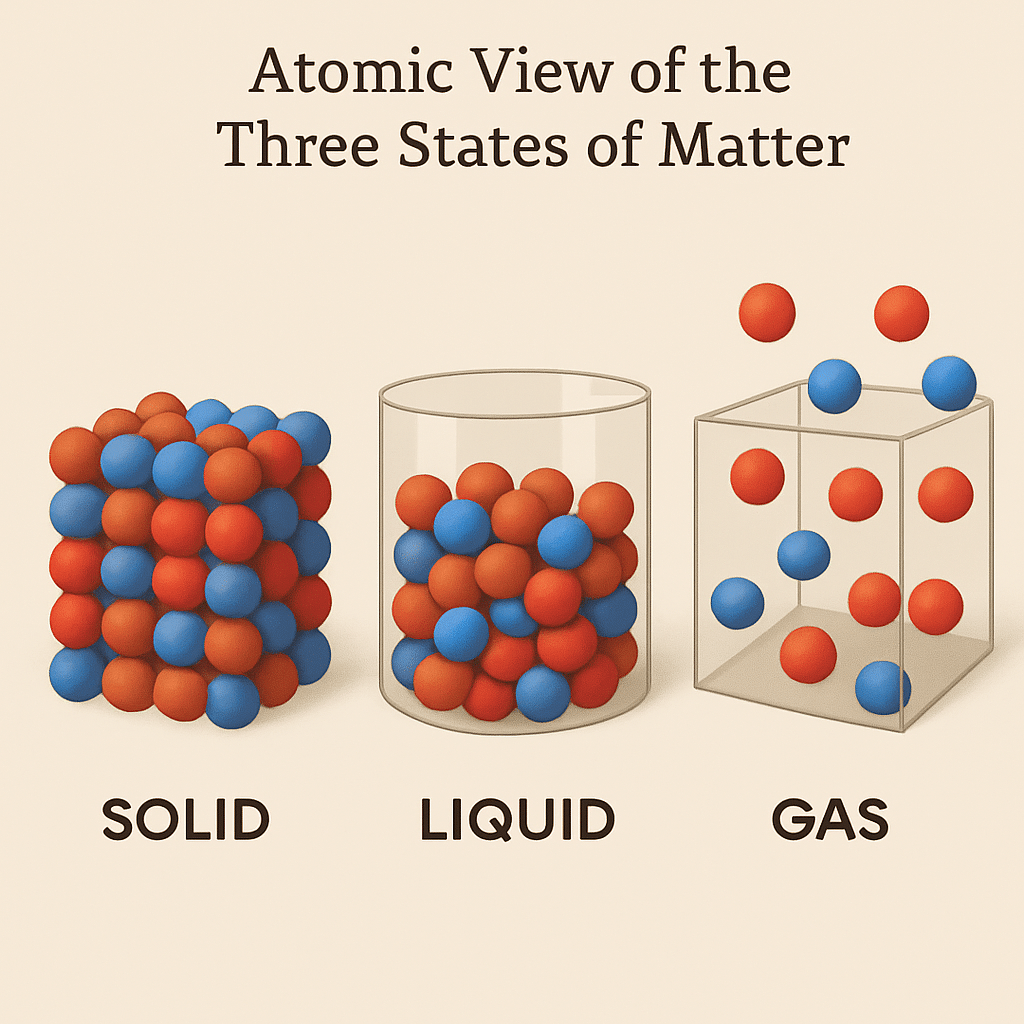
Matter around us exists in three different states:
Solid
Liquid
Gas
1.3.1 The Solid State
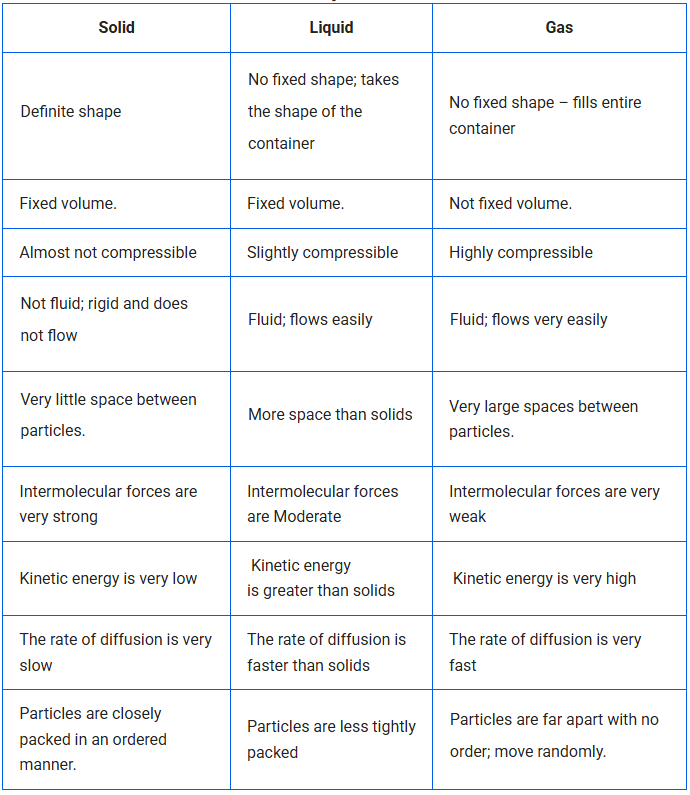
Solids have a definite shape and a definite volume.
Solids are rigid.
- Solids have negligible compressibility.
Solids have high density.
- The intermolecular spaces between the particles of solids are very small.
The force of attraction between particles is very strong.
Solids cannot flow.
- The rate of diffusion in solids is low.
Exceptions (still considered solids):
Rubber band: It changes shape when stretched and returns to its original shape.
Salt or sugar: It takes the shape of a jar, but each crystal has a fixed shape.
Sponge: It can be compressed due to air holes, but it is a solid.
1.3.2 The Liquid State
Liquids have no fixed shape but have a fixed volume.
They take the shape of the container in which they are kept.
Liquids flow, so they are called fluids.
- The intermolecular spaces between the particles of liquids are greater than solids.
The intermolecular force of attraction is less than solids.
Liquids show less compressibility than gases.
- Solids, liquids and gases can diffuse into liquids.
- The rate of diffusion of liquids is higher than that of solids.
Gases like oxygen and carbon dioxide dissolve in water and are essential for aquatic life.
1.3.3 The Gaseous State
Gases have neither a definite shape nor a definite volume.
Gases fill the entire container in which they are kept.
They are highly compressible. The liquefied petroleum gas (LPG) in cylinders is compressed gas. Compressed Natural gas (CNG) is used as fuel these days in vehicles.
Particles move randomly at high speed.
- The intermolecular spaces between the particles of gases are maximum.
- The intermolecular force of attraction between particles is very weak.
Gases diffuse very fast because of the large space and high speed of particles.
The pressure of a gas is due to the force exerted by particles on the walls of the container.
- The rate of diffusion in gases is maximum.
Differences Between Solid, Liquid, and Gas
1.4 Can Matter Change Its State?
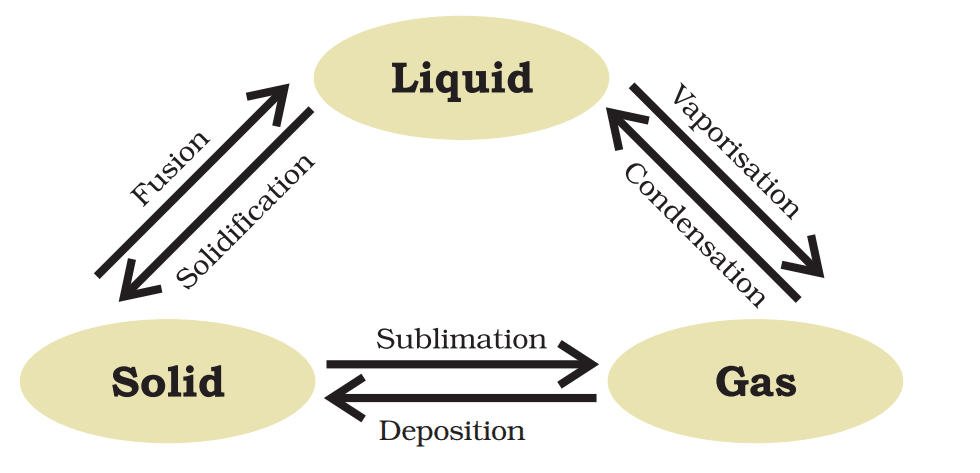
Water can exist in three states:
Solid as ice
Liquid as water
Gas as water vapour
1.4.1 Effect of Change of Temperature
When we increase the temperature of solids, the kinetic energy of particles increases.
This makes particles vibrate faster and overcome the force of attraction. The
particles leave their fixed positions and start moving more freely. Eventually, the solid changes into a liquid.
- The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
Melting (Solid → Liquid)
The melting point of ice is 273.15 K (or 0°C).
The process of melting is also called fusion.
During melting, the temperature remains constant until the entire solid is converted into liquid. The heat supplied is used to break the force of attraction – this is called latent heat.
👉Latent Heat:
Latent heat is the hidden heat energy absorbed or released by a substance during a change of state (like melting or boiling) without any change in temperature. It is used to break the force of attraction between particles during the change of state.
👉 Latent Heat of Fusion:
The amount of heat energy required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Boiling (Liquid → Gas)
On further heating, the liquid starts boiling and changes into a gas.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
The boiling point of water is 373 K (100°C).
Boiling is a bulk phenomenon, where particles from the entire liquid gain energy to become vapour.
👉 Latent Heat of Vaporisation:
The amount of heat energy required to change 1 kg of a liquid into a gas at atmospheric pressure at its boiling point is known as the latent heat of fusion.
👉 Sublimation:
A change of state directly from solid to gas without changing into a liquid state is called sublimation. Example: Camphor
👉 Deposition:
The direct change of gas to solid without changing into liquid is called deposition.
1.4.2 Effect of Change of Pressure
We know the particles of gases are far apart. By applying pressure, we can bring them closer.
Gases can be liquefied by increasing pressure and lowering temperature.
Dry Ice (Solid CO₂)
When the pressure is reduced to 1 atmosphere, solid CO₂ changes directly into gas without becoming liquid. Therefore, solid carbon dioxide is called dry ice.
1.5 Evaporation
We know that liquids can change into vapour by boiling. But sometimes liquids also change into vapour at room temperature, without boiling. This process is called evaporation.
What is Evaporation?
Evaporation is the change of liquid into vapour at any temperature below its boiling point.
It is a surface phenomenon.
Only the particles at the surface of the liquid gain enough energy to overcome the forces of attraction and change into vapour.
These particles have higher kinetic energy.
1.5.1 Factors Affecting Evaporation
Several factors affect the rate of evaporation. These are:
An increase in surface area:
More surface area → faster evaporation
- If the surface area is increased, the rate of evaporation increases.
Example: Clothes dry faster when spread out.
An increase in temperature:
Higher temperature → faster evaporation
- So, with the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
a decrease in humidity:
Humidity is the amount of water vapour in the air.
More humidity → slower evaporation
- When the amount of water in the air is already high, the rate of evaporation decreases.
An increase in wind speed:
More wind speed → faster evaporation
Wind blows away the water vapour, allowing more particles to evaporate.
Example: Clothes dry faster on a windy day.
1.5.2 How Does Evaporation Cause Cooling?
During evaporation, the particles absorb heat energy from the surroundings to convert into vapour. This removes heat from the surface and causes cooling.
Examples from Daily Life:
Acetone or perfume on the palm feels cold because it evaporates quickly and absorbs heat.
Sprinkling water on the roof cools the surface due to evaporation.
Why should we wear cotton clothes in summer?
We should wear cotton clothes in summer because cotton is a good absorber of water, so it absorbs sweat and exposes it to the atmosphere. This helps the sweat evaporate faster. During evaporation, the particles at the surface of the liquid absorb heat energy from the surroundings or body surface and change into vapour. This removes heat from the surface and causes cooling.
Why do we see water droplets on the outer surface of a glass containing ice-cold water?
We see water droplets on the outer surface of a glass containing ice-cold water because the water vapour present in the air comes in contact with the cold surface of the glass, loses energy, and gets converted to liquid state. These droplets are formed by condensation of water vapour from the air.
NCERT Notes for Class 9 Science
- Chapter 1 Matter in Our Surroundings Class 9 Notes
- Chapter 2 Is Matter Around Us Pure Class 9 Notes
- Chapter 3 Atoms and Molecules Class 9 Notes
- Chapter 4 Structure of the Atom Class 9 Notes
- Chapter 5 The Fundamental Unit of Life Class 9 Notes
- Chapter 6 Tissues Class 9 Notes
- Chapter 7 Diversity in Living Organisms Class 9 Notes
- Chapter 8 Motion Class 9 Notes
- Chapter 9 Force and Laws of Motion Class 9 Notes
- Chapter 10 Gravitation Class 9 Notes
- Chapter 11 Work, Power And Energy Class 9 Notes
- Chapter 12 Sound Class 9 Notes
- Chapter 13 Why Do we Fall ill Class 9 Notes
- Chapter 14 Natural Resources Class 9 Notes
- Chapter 15 Improvement in Food Resources Class 9 Notes
