Atoms and Molecules Class 9 Notes Science Chapter 3
Here we have provided summary and revision notes for Class 9 Science Chapter 3 – Atoms and Molecules. These notes include key points, short revision notes, diagrams, and explanations from the entire chapter. If you are a Class 9 student studying from the NCERT Science textbook, this chapter is very important.
After reading the lesson, you can use these notes to revise and remember the main concepts easily. You will find everything about Chapter 3: Atoms and Molecules in one place.
Table of Contents
Introduction
Have you ever wondered what makes up everything around us? From the water we drink to the air we breathe, everything in our universe is made up of tiny particles called atoms and molecules. This chapter will take you on an exciting journey to understand these building blocks of matter.
Historical Background
The idea that matter is made of tiny particles is not new. Let’s explore how this concept developed:
Ancient Indian Philosophy (500 BC):
- An Ancient Indian philosopher Maharishi Kanad, postulated that if we keep dividing matter, we will reach the smallest particles called ‘Parmanu’.
- Pakudha Katyayama added that these particles exist in combined form to make matter.
Ancient Greek Philosophy:
Greek philosophers Democritus and Leucippus also proposed that matter is made up of tiny indivisible particles called atoms.
These ideas were just philosophical without experiments.
Modern Development:
- Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws of chemical combination.
- John Dalton gave the atomic theory in 1808
Laws of Chemical Combination
Before understanding atoms, we need to learn two important laws discovered by Lavoisier and Joseph L. Proust:
1. Law of Conservation of Mass
This law was proposed by Lavoisier.
Law of conservation of mass states that “Mass can neither be created nor destroyed in a chemical reaction.“
Explanation:
- During any chemical reaction, the total mass of reactants = Total mass of products
- Matter only changes from one form to another
- No mass is lost or gained
Example:
A + B → AB
Mass of A + Mass of B = Mass of AB
2. Law of Constant Proportions (Law of Definite Proportions)
This law was proposed by Joseph Proust.
It states that: “In a chemical substance, the elements are always present in definite proportions by mass.”
Examples:
- In water (H₂O): Hydrogen : Oxygen = 1 : 8 (by mass)
- In ammonia (NH₃): Nitrogen : Hydrogen = 14 : 3 (by mass)
- In carbon dioxide (CO₂): Carbon : Oxygen = 3 : 8 (by mass)
This means no matter how water is prepared or where it comes from, the ratio of hydrogen to oxygen will always be 1:8.
Dalton’s Atomic Theory
John Dalton gave the atomic theory in 1808. Dalton’s atomic theory was based on the laws of chemical combination. His theory provided an explanation for the law of conservation of mass and the law of definite proportions.
Postulates of Dalton’s Atomic Theory:
- All matter is made of very tiny particles called atoms, which participate in chemical reactions.
- Atoms are indivisible particles and cannot be created or destroyed in chemical reactions.
- Atoms of the same element are identical in mass and chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Significance:
- It explained law of conservation of mass (atoms cannot be created or destroyed)
- It explained law of constant proportions (atoms combine in fixed ratios)
What is an Atom?
Atoms are the basic building blocks of matter.
Definition: An atom is the smallest particle of an element that cannot usually exist independently and retains all chemical properties of that element.
Examples:- One H atom, one O atom, one Fe atom.
How big are atoms?
- Atoms are extremely small.
- Atomic radius is measured in nanometers (nm)
- 1 nanometer = 1/10⁹ meters or 1 m = 10⁹ nm
Size Comparison:
| Object | Size (in meters) |
|---|---|
| Hydrogen atom | 10⁻¹⁰ |
| Water molecule | 10⁻⁹ |
| Grain of sand | 10⁻⁴ |
| Ant | 10⁻³ |
| Apple | 10⁻¹ |
What is Elements?
An element is a pure substance made of only one type of atom. It cannot be broken down into simpler substances by any chemical reaction.
Examples:- Hydrogen (H₂ gas), Oxygen (O₂ gas), Iron (Fe metal), Gold (Au).
Modern Symbols of Elements:
Dalton was the first scientist to use symbols for elements. Dalton used simple drawings. Now, we use symbols decided by IUPAC (International Union of Pure and Applied Chemistry).
Rules for writing symbols:
- First letter is always capital
- Second letter (if any) is always small
- Maximum two letters in a symbol
Examples:
- Hydrogen = H
- Helium = He
- Aluminum = Al (not AL)
- Cobalt = Co (not CO)
Some symbols from Latin names:
- Iron = Fe (from Latin ‘ferrum’)
- Sodium = Na (from Latin ‘natrium’)
- Potassium = K (from Latin ‘kalium’)
- Gold = Au (from Latin ‘aurum’)
Atomic Mass:
Definition: The relative atomic mass of an element is the average mass of its atoms compared to 1/12th the mass of a carbon-12 atom.
Atomic Mass Unit (u):
- Earlier called ‘amu’ (atomic mass unit)
- Now called ‘u’ (unified mass)
- 1 u = 1/12th the mass of one carbon-12 atom
Atomic Masses of first 20 elements
| Atomic Number | Element Name | Symbol | Atomic Mass (Rounded off) |
|---|---|---|---|
| 1 | Hydrogen | H | 1 |
| 2 | Helium | He | 4 |
| 3 | Lithium | Li | 7 |
| 4 | Beryllium | Be | 9 |
| 5 | Boron | B | 11 |
| 6 | Carbon | C | 12 |
| 7 | Nitrogen | N | 14 |
| 8 | Oxygen | O | 16 |
| 9 | Fluorine | F | 19 |
| 10 | Neon | Ne | 20 |
| 11 | Sodium | Na | 23 |
| 12 | Magnesium | Mg | 24 |
| 13 | Aluminium | Al | 27 |
| 14 | Silicon | Si | 28 |
| 15 | Phosphorus | P | 31 |
| 16 | Sulphur | S | 32 |
| 17 | Chlorine | Cl | 35.5 |
| 18 | Argon | Ar | 40 |
| 19 | Potassium | K | 39 |
| 20 | Calcium | Ca | 40 |
How do Atoms Exist?
Atoms of most elements are not able to exist independently. Atoms form molecules and ions, which combine in large numbers to form matter that we can see, feel or touch.
Exception: Noble gases (He, Ne, Ar, Kr, Xe, Rn) can exist as single atoms because they are chemically unreactive.
What is a Molecule?
A molecule is a group of two or more atoms that are chemically bonded together.
Definition: A molecule is the smallest particle of an element or compound that can exist independently and shows all properties of that substance.
Formation: Two or more atoms of the same element or of different elements can join together to form molecules.
Types of Molecules:
1. Molecules of Elements:
The molecules of an element are made up of the same kind of atoms.
Examples:
- Monoatomic: Argon (Ar), Helium (He)
- Diatomic : Hydrogen (H₂), Oxygen (O₂), Nitrogen (N₂), Chlorine (Cl₂)
- Triatomic: Ozone (O₃)
- Tetratomic: Phosphorus (P₄)
- Polyatomic: Sulphur (S₈)
What is Atomicity?
Definition: The number of atoms present in one molecule of an element is called its atomicity.
Example:- O₂ → atomicity 2, P₄ → atomicity 4, S₈ → atomicity 8
| Element | Formula | Atomicity |
|---|---|---|
| Argon | Ar | Monoatomic (1) |
| Hydrogen | H₂ | Diatomic (2) |
| Oxygen | O₂ | Diatomic (2) |
| Ozone | O₃ | Triatomic (3) |
| Phosphorus | P₄ | Tetratomic (4) |
| Sulphur | S₈ | Polyatomic (8) |
2. Molecules of Compounds:
Atoms of different elements join together in definite proportions to form molecules of compounds.
Examples:
- Water (H₂O) – 2 hydrogen + 1 oxygen
- Carbon dioxide (CO₂) – 1 carbon + 2 oxygen
- Ammonia (NH₃) – 1 nitrogen + 3 hydrogen
- Methane (CH₄) – 1 carbon + 4 hydrogen
Ions and Chemical Formulae
What is an Ion?
Definition: An ion is an atom or group of atoms that carries an electric charge.
Types of Ions:
1. Cation (Positive Ion):
- A positively charged ion is called a ‘cation’.
- Formed when an atom loses electrons
- Examples: Na⁺, Ca²⁺, Al³⁺
2. Anion (Negative Ion):
- A negatively charged ion is called an ‘anion’.
- Formed when an atom gains electrons
- Examples: Cl⁻, O²⁻, N³⁻, S²⁻
3. Polyatomic Ions:
- A group of atoms carrying a charge is known as a polyatomic ion.
- Carry fixed charge
Examples:
Hydroxide → OH⁻
Ammonium → NH₄⁺
Carbonate → CO₃²⁻
Sulphate → SO₄²⁻
Common Positive Ions (Cations)
| Valency | Name of Ion | Symbol |
|---|---|---|
| 1 | Sodium | Na⁺ |
| 1 | Potassium | K⁺ |
| 1 | Silver | Ag⁺ |
| 1 | Copper (I) | Cu⁺ |
| 2 | Magnesium | Mg²⁺ |
| 2 | Calcium | Ca²⁺ |
| 2 | Zinc | Zn²⁺ |
| 2 | Iron (II) | Fe²⁺ |
| 2 | Copper (II) | Cu²⁺ |
| 3 | Aluminium | Al³⁺ |
| 3 | Iron (III) | Fe³⁺ |
Common Negative Ions (Anions)
| Valency | Name of Ion | Symbol |
|---|---|---|
| 1 | Hydride | H⁻ |
| 1 | Chloride | Cl⁻ |
| 1 | Bromide | Br⁻ |
| 1 | Iodide | I⁻ |
| 2 | Oxide | O²⁻ |
| 2 | Sulphide | S²⁻ |
| 3 | Nitride | N³⁻ |
Common Polyatomic Ions
| Valency | Name of Ion | Symbol |
|---|---|---|
| 1 | Ammonium | NH₄⁺ |
| 1 | Hydroxide | OH⁻ |
| 1 | Nitrate | NO₃⁻ |
| 1 | Hydrogen carbonate | HCO₃⁻ |
| 2 | Carbonate | CO₃²⁻ |
| 2 | Sulphite | SO₃²⁻ |
| 2 | Sulphate | SO₄²⁻ |
| 3 | Phosphate | PO₄³⁻ |
Valency:
Definition: The combining power or capacity of an element is known as its valency.
In simple terms, the number of electrons lost, gained, or shared = Valency.
Examples
- Hydrogen (H) → 1 electron in outer shell → valency 1.
- Oxygen (O) → needs 2 more electrons to complete shell → valency 2.
- Nitrogen (N) → needs 3 electrons → valency 3.
- Carbon (C) → can share 4 electrons → valency 4.
- Sodium (Na) → loses 1 electron → valency 1.
- Calcium (Ca) → loses 2 electrons → valency 2.
Valency of Common Elements
| Element | Symbol | Valency |
|---|---|---|
| Hydrogen | H | 1 |
| Helium (noble gas) | He | 0 (already stable) |
| Lithium | Li | 1 |
| Sodium | Na | 1 |
| Potassium | K | 1 |
| Magnesium | Mg | 2 |
| Calcium | Ca | 2 |
| Aluminium | Al | 3 |
| Carbon | C | 4 |
| Nitrogen | N | 3 |
| Oxygen | O | 2 |
| Sulphur | S | 2 |
| Chlorine | Cl | 1 |
| Iron (II) | Fe²⁺ | 2 |
| Iron (III) | Fe³⁺ | 3 |
| Copper (I) | Cu⁺ | 1 |
| Copper (II) | Cu²⁺ | 2 |
| Zinc | Zn | 2 |
| Silver | Ag | 1 |
| Phosphorus | P | 3 or 5 |
Writing Chemical Formulae
Definition: A chemical formula represents the composition of a compound using symbols and numbers.
Rules for Writing Chemical Formulae:
- The valencies of the atoms/ions must balance each other. i.e., Total positive charge = total negative charge.
- In compounds of metals and non-metals: Write the metal (positive ion) first and the non-metal (negative ion) second. Example: NaCl (Sodium chloride), CaO (Calcium oxide).
If a polyatomic ion appears more than once, put it in brackets. Example: Ca(OH)₂, (NH₄)₂SO₄.
If it appears only once, no brackets are needed. Example: NaOH.
Steps to Write Chemical Formula:
- Write the symbols of the elements/ions.
- Write their valencies below the symbols.
- Criss-cross the valencies → write them as subscripts.
- If needed, simplify the ratio.
Examples
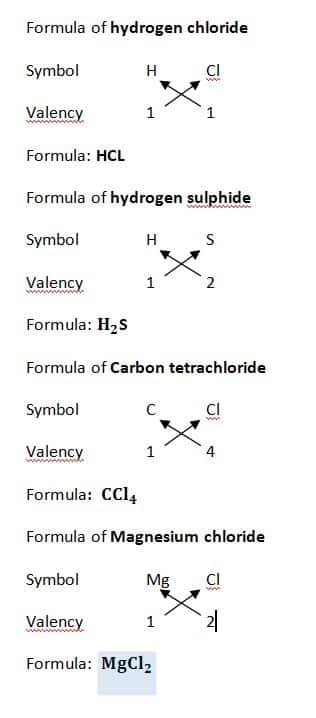
Similarly
Aluminium oxide
- Al (3), O (2)
- Criss-cross → Al₂O₃
- Formula = Al₂O₃
Calcium oxide
- Ca (2), O (2)
- Simplify 2:2 → 1:1
- Formula = CaO
Calcium hydroxide
- Ca (2), OH (1)
- Criss-cross → Ca(OH)₂
- Formula = Ca(OH)₂
Sodium carbonate
- Na (1), CO₃ (2)
- Criss-cross → Na₂CO₃
- Formula = Na₂CO₃
Ammonium sulphate
- NH₄ (1), SO₄ (2)
- Criss-cross → (NH₄)₂SO₄
- Formula = (NH₄)₂SO₄
Molecular Mass and Formula Unit Mass
Molecular Mass:
Definition: The molecular mass of a substance is the sum of the atomic masses of all atoms present in a molecule.
- It is therefore the relative mass of a molecule expressed in atomic mass units (u).
Calculation Method:
Write the molecular formula
Count the number of each type of atom
Multiply atomic mass by number of atoms
Add all values
Examples:
Water (H₂O):
H = 1 u, O = 16 u
Molecular mass = (2 × 1) + (1 × 16) = 18 u
Nitric acid (HNO₃):
H = 1 u, N = 14 u, O = 16 u
Molecular mass = 1 + 14 + (3 × 16) = 63 u
Formula Unit Mass:
It is used for ionic compounds.
Definition: The formula unit mass is the sum of the atomic masses of all atoms in a formula unit of an ionic compound.
Examples:
Sodium chloride (NaCl):
Na = 23 u, Cl = 35.5 u
Formula unit mass = 23 + 35.5 = 58.5 u
Calcium chloride (CaCl₂):
Ca = 40 u, Cl = 35.5 u
Formula unit mass = 40 + (2 × 35.5) = 40 + 71 = 111 u
Mole Concept
What is a Mole?
Definition: A mole is the amount of substance that contains 6.022 × 10²³ particles (atoms, molecules, or ions).
This number (6.022 × 10²³) is called Avogadro’s number (N0)
Key Points
- 1 mole of atoms = 6.022 × 10²³ atoms.
- 1 mole of molecules = 6.022 × 10²³ molecules.
- 1 mole of ions = 6.022 × 10²³ ions.
- 1 mole of a substance = Its atomic/molecular mass in grams
Examples:
- 1 mole of carbon atoms = 12g = 6.022 × 10²³ atoms
- 1 mole of oxygen molecules = 32g = 6.022 × 10²³ molecules
- 1 mole of water = 18g = 6.022 × 10²³ molecules
What is a Molar Mass?
Molar mass is the mass of 1 mole of a substance.
Difference Between Molar Mass and Molecular Mass
| Basis | Molecular Mass | Molar Mass |
|---|---|---|
| Definition | It is the sum of the atomic masses of all atoms present in a molecule. | It is the mass of one mole (6.022 × 10²³ particles) of a substance. |
| Unit | Atomic mass unit (u or amu) | Gram per mole (g/mol) |
| Example | Molecular mass of H₂O = (2×1) + 16 = 18 u | Molar mass of H₂O = 18 g/mol |
Important Formulas:
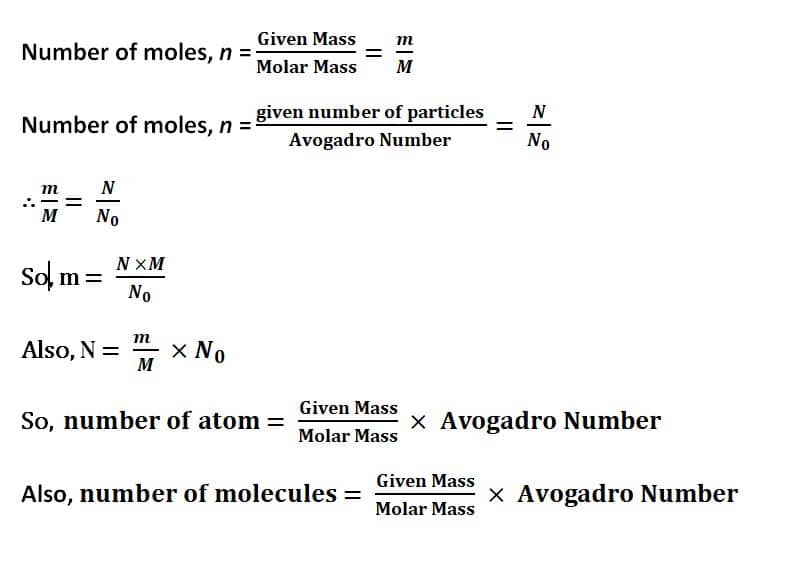
Important Numerical Problems
Question 1: Calculate the number of moles in 10g of water.
Solution:
- Molecular mass of H₂O = 18u
- Molar mass = 18g
- Given mass = 10g
- Number of moles = 10/18 = 0.56 moles
Question 2: How many molecules are present in 4g of oxygen gas?
Solution:
- Molecular mass of O₂ = 32u
- Number of moles = 4/32 = 0.125 moles
- Number of molecules = 0.125 × 6.022 × 10²³ = 7.53 × 10²² molecules
Question 3: What is the mass of 0.2 moles of carbon dioxide?
Solution:
- Molecular mass of CO₂ = 44u
- Mass = 0.2 × 44 = 8.8g
Question 4: Calculate the formula unit mass of CaCO₃.
Solution:
- Ca: 40 × 1 = 40u
- C: 12 × 1 = 12u
- O: 16 × 3 = 48u
- Total = 100u
Key Terms and Definitions
| Term | Definition |
|---|---|
| Atom | Smallest particle of an element that retains its properties |
| Molecule | Smallest particle that can exist independently |
| Ion | Charged atom or group of atoms |
| Cation | Positively charged ion |
| Anion | Negatively charged ion |
| Valency | Combining capacity of an element |
| Atomicity | Number of atoms in a molecule |
| Molecular Mass | Sum of atomic masses in a molecule |
| Formula Unit Mass | Sum of atomic masses in an ionic compound |
| Mole | 6.022 × 10²³ particles |
| Avogadro’s Number | 6.022 × 10²³ |
| Chemical Formula | Symbolic representation of a compound |
Important Questions for Exam
Very Short Answer Questions (1 Mark):
- Define atom.
- What is the atomicity of ozone?
- Write the chemical formula of calcium oxide.
- Define molecular mass.
- What is Avogadro’s number?
Short Answer Questions (2 Marks):
- State the law of conservation of mass.
- Define valency with an example.
- What are polyatomic ions? Give two examples.
- Calculate the molecular mass of CO₂.
- How many atoms are present in H₂SO₄?
Short Answer Questions (3 Marks):
- State and explain Dalton’s atomic theory.
- Differentiate between molecular mass and formula unit mass.
- Calculate the number of moles in 44g of CO₂.
- Write the chemical formulae of: (a) Sodium carbonate (b) Ammonium sulphate
- Explain the mole concept with an example.
Long Answer Questions (5 Marks):
- Explain the laws of chemical combination with examples.
- What are molecules? Explain the types of molecules with examples.
- Describe how chemical formulae are written. Give five examples.
- Define mole concept. How many molecules are present in 9g of water?
- Write the differences between atoms and molecules. Give examples.
Tips for Exam Success:
- Learn chemical symbols of first 20 elements
- Practice numerical problems regularly
- Memorize common valencies and polyatomic ions
- Understand concepts rather than rote learning
- Draw diagrams wherever possible
- Practice writing chemical formulae daily
- Solve previous year questions
- Make flashcards for important formulas
These notes are based on NCERT Class 9 Science textbook and are designed to help students understand the fundamental concepts of atoms and molecules. Regular practice of numerical problems and thorough understanding of concepts will ensure success in examinations.
NCERT Notes for Class 9 Science
- Chapter 1 Matter in Our Surroundings Class 9 Notes
- Chapter 2 Is Matter Around Us Pure Class 9 Notes
- Chapter 3 Atoms and Molecules Class 9 Notes
- Chapter 4 Structure of the Atom Class 9 Notes
- Chapter 5 The Fundamental Unit of Life Class 9 Notes
- Chapter 6 Tissues Class 9 Notes
- Chapter 7 Diversity in Living Organisms Class 9 Notes
- Chapter 8 Motion Class 9 Notes
- Chapter 9 Force and Laws of Motion Class 9 Notes
- Chapter 10 Gravitation Class 9 Notes
- Chapter 11 Work, Power And Energy Class 9 Notes
- Chapter 12 Sound Class 9 Notes
- Chapter 13 Why Do we Fall ill Class 9 Notes
- Chapter 14 Natural Resources Class 9 Notes
- Chapter 15 Improvement in Food Resources Class 9 Notes
